Second Ionization Energy Chart. H 1st H 2nd H 3rd. Thats a lot of energy.
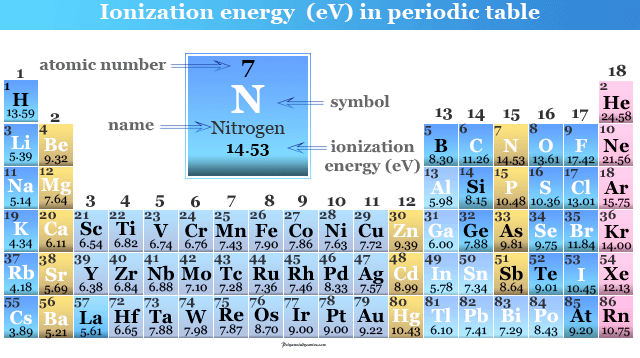
2nd ionization energy - The energy required to remove a second electron from a singly charged gaseous cation. 9 rows ionization energy shows a similar trend but shifted. The 2nd ionization energy of the element M is a measure of the energy required to remove one electron from one mole of the gaseous ion M Image showing periodicity of the chemical elements for ionization energy.
Second ionization energy is the energy needed to remove a second electron from an atom after one has already been removed.
9 rows ionization energy shows a similar trend but shifted. Second ionisation energy is defined by the equation. Because of the enhanced stability of half-filled and fully filled orbitals removal of electrons from such systems will have relatively higher ionization than other atoms and ions. The third ionization energy is the energy required to form 3 cations.